PRODUCT SEARCH
Enquiries
If you have any questions about products featured on this site, or on products that you cannot find on this site, please contact us and we will be happy to assist you in any way that we can. We have a team of technical sales specialists who are waiting to hear from you!
Contact UsHome > Life Science Solutions > Purification and Downstream Processing > Downstream Processing Challenges
Downstream Process Challenges
DE (Kieselguhr) has long been used in beer filtration, but using it in heavily regulated and sensitive processes is difficult because the standard grades of DE are simply too volatile and of insufficient purity from source to source. As a result, to meet the challenge, a highly purified DE that meets USP monographs was created.
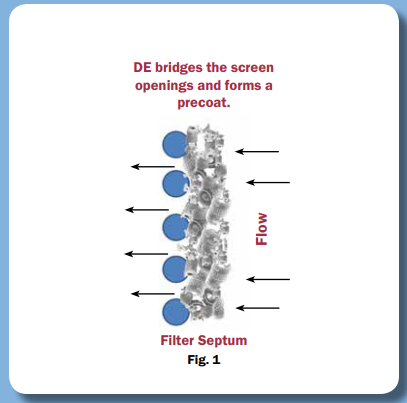
We sell our PurifiDE® line of highly purified DE for use in cGMP production as it satisfies the requirements of high purity, consistency, source control and low extractables. We are well-positioned to assist you in making use of this powerful and cost-effective processing approach, thanks to our portfolio of downstream Bioprocess solutions.
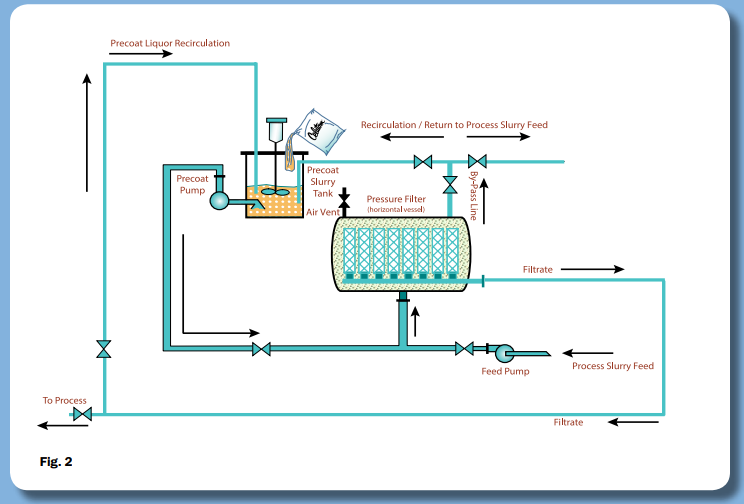
PurifiDE® in conjunction with our Single Use Filtration solution (SU Filtration) offers a scalable single or multi-cycle filtration solution for cell removal post-fermentation and reduction of host cell proteins (HCP) – download our whitepaper on the application below:
Purification and Downstream Processing1 items found

|
PurifiDE® XG
The PurifiDE® range of Purified USP-NF Diatomaceous Earth Filter Aids called "Bulk Pharmaceutical Excipient Filter Aid” (BPEFA) have been created for use (21 CFR Part 210.3) in the filtering of human and animal drug products via chemical or biological routes. PurifiDE® XG BPEFAs are manufactured in accordance with cGMP Practices for Bulk Pharmaceutical Excipients (USP40-NF35 <1078>). Enquire about this product |
View details |
